Background: Polycythemia vera (PV) is a chronic myeloproliferative neoplasm characterized by erythrocytosis defined by an acquired increase in hemoglobin (Hb)/hematocrit (HCT). Disease complications are associated with increased blood viscosity, cardiovascular/thrombotic events and iron deficiency (Marchioli et al. N Engl J Med. 2013;368:22-33). Most patients with PV are iron deficient and therapeutic phlebotomy may exacerbate iron deficiency (Ginzburg et al. Leukemia. 2018;32:2105-16).
Methods:PTG-300-08 (clinicaltrials.gov NCT04767802, PACIFIC study) is an open-label, 52-week, phase 2 study that investigated the efficacy and safety of rusfertide in Asian patients with poor hematocrit (HCT) control as noted by HCT >48%. Treatment was initiated with 40 mg rusfertide subcutaneously (SC) twice a week. Once HCT was <45%, the treatment was changed to 40 mg once a week with subsequent dose adjustments allowed to maintain HCT <45%. In addition to HCT and erythrocyte morphology (mean corpuscular volume, or MCV), biomarkers reflective of iron biology, such as hepcidin, erythroferrone (ERFE), erythropoietin (EPO), and soluble transferrin receptor (sTfR) were measured at baseline, Week 4, 16, and 32 following initiation of rusfertide treatment. To examine the effect of rusfertide on serum iron, patients were stratified by baseline ferritin levels to examine patients who were iron deficient (ferritin ≤20 µg/L) compared to patients who had normal iron (ferritin >20 µg/L).
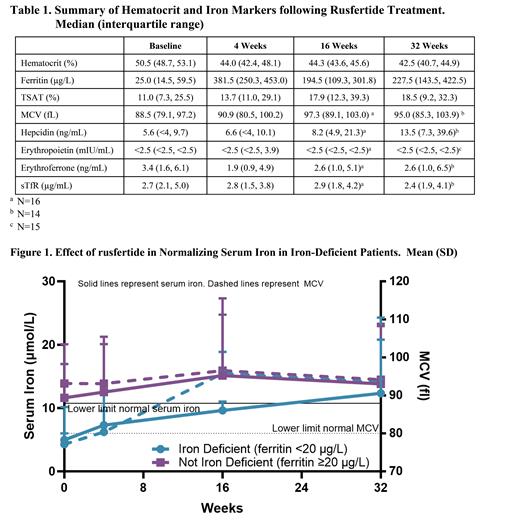
Results: Twenty patients (15 males, 5 females), average age 56.7 (SD, 9.12) years and average weight 65 (10.9) kg were enrolled. Thirteen patients (65%) were low risk and 7 (35%) were high risk, all based on age. No patients had a prior history of thromboembolic events. Mean duration since PV diagnosis was 4.2 (3.83) years. Rusfertide rapidly and robustly reduced mean HCT and sustained HCT <45% for the duration of treatment, and ferritin, TSAT and MCV increased (Table 1). At baseline, 30% of subjects had serum ferritin levels below 20 µg/L, with all subjects achieving and sustaining ferritin levels above 20 µg/L by Week 4 following initiation of rusfertide treatment. Table 1 also summarizes the biomarkers related to iron indices. Median hepcidin concentrations increased, median EPO concentration was below and remained below the limit of detection, and ERFE concentration decreased modestly with rusfertide dosing consistent with reduced erythropoietic drive. Among patients with evidence of baseline iron deficiency (ferritin <20 µg/L), rusfertide normalized iron levels while iron levels remained stable in rusfertide patients with normal iron levels at baseline (Figure 1). Similarly, patients with iron deficiency had low MCV at baseline (Figure 1) which increased following initiation of rusfertide treatment. In patients with normal iron levels, MCV remained stable in the normal range (>80 fl).
Conclusions: Treatment with rusfertide resulted in rapid, robust, and sustained reduction in HCT levels in PV patients with elevated HCT levels at baseline. Treatment resulted in normalization of iron parameters as noted by increases in ferritin, and normalization of serum iron and MCV in patients who were iron deficient at baseline. Improvements in iron deficiency following rusfertide are suggestive of clinical benefits and merit further investigation. This analysis confirms the general understanding that managing iron deficiency in PV remains an unmet need.
Disclosures
Ginzburg:Protagonist Therapeutics, Inc.: Consultancy, Research Funding. Chew:Protagonist Therapeutics, Inc.: Research Funding. Modi:Protagonist Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Gupta:Protagonist Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Molina:Protagonist Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Modi:Protagonist Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company.